Lithium-ion batteries have been widely used in consumer electronics, electric vehicles, energy storage, and other fields due to their high specific energy and power density, long cycle life, and environmental friendliness. As the power source for new energy vehicles, lithium-ion batteries still have many problems in practical applications. For example, their energy density decreases significantly under low-temperature conditions, and their cycle life is also affected accordingly, which seriously restricts the large-scale application of lithium-ion batteries.
At present, researchers still have disputes about the main factors causing the poor low-temperature performance of lithium-ion batteries, but the reasons can be summarized into the following three aspects:
1.Under low temperatures, the viscosity of the electrolyte increases, and its conductivity decreases;
2.The impedance of the electrolyte/electrode interface film and the charge transfer impedance increase;
3.The migration rate of lithium ions in the bulk of the active material decreases. These factors lead to intensified electrode polarization and reduced charge-discharge capacity at low temperatures.
In addition, during low-temperature charging, especially high-rate charging at low temperatures, lithium metal will precipitate and deposit on the negative electrode. The deposited metallic lithium is prone to irreversible reactions with the electrolyte, consuming a large amount of electrolyte. At the same time, it further increases the thickness of the SEI film, leading to a further increase in the impedance of the film on the negative electrode surface of the battery and a re-intensification of battery polarization. Ultimately, this will greatly damage the low-temperature performance, cycle life, and safety performance of the battery.
This article systematically discusses the main factors affecting the low-temperature performance of lithium-ion batteries from three aspects: positive electrode materials, electrolytes, and negative electrode materials, and proposes effective methods to improve the low-temperature performance of lithium-ion batteries.
1. Cathode Materials
Cathode materials are one of the key materials for manufacturing lithium-ion batteries, and their performance directly affects various indicators of the battery. The structure of the material has an important impact on the low-temperature performance of lithium-ion batteries.
LiFePO₄ with an olivine structure has the advantages of high discharge specific capacity, stable discharge platform, stable structure, excellent cycle performance, and abundant raw materials, making it a mainstream cathode material for lithium-ion power batteries. However, lithium iron phosphate belongs to the Pnma space group, where P occupies the tetrahedral position, transition metal M occupies the octahedral position, and Li atoms form a migration channel along the one-dimensional direction of the [010] axis. This one-dimensional ion channel causes lithium ions to be deintercalated or intercalated in an orderly and single manner, which seriously affects the diffusion ability of lithium ions in this material. Especially at low temperatures, the diffusion of lithium ions in the bulk is further hindered, resulting in increased impedance, more severe polarization, and poor low-temperature performance.
Nickel-cobalt-manganese-based LiNiₓCoᵧMn₁₋ₓ₋ᵧO₂ is a new type of solid solution material developed in recent years, with a single-phase layered structure similar to LiCoO₂ (α-NaFeO₂ type). This material has important advantages such as high reversible specific capacity, good cycle stability, and moderate cost. It has also been successfully applied in the field of power batteries, and the scale of application has developed rapidly. However, there are still some problems that need to be solved urgently, such as low electronic conductivity, poor high-rate stability, and especially the deterioration of the high and low-temperature performance of the material with the increase of nickel content.
Lithium-rich manganese-based layered cathode materials have higher discharge specific capacity and are expected to become the next-generation cathode materials for lithium-ion batteries. However, lithium-rich manganese-based materials have many problems in practical applications: high first irreversible capacity, easy transformation from a layered structure to a spinel structure during charge and discharge, which causes the Li⁺ diffusion channels to be blocked by the migrated transition metal ions, resulting in severe capacity fading. At the same time, their own ion and electronic conductivity are not good, leading to poor rate performance and low-temperature performance.
The mainstream methods to improve the ion diffusion performance of cathode materials at low temperatures are as follows:
1.1 Surface Coating with Materials of Excellent Conductivity on the Bulk of Active Materials
This method can improve the conductivity of the cathode material interface, reduce the interface impedance, and at the same time reduce the side reactions between the cathode material and the electrolyte, and stabilize the material structure.
Rui et al. studied the low-temperature performance of carbon-coated LiFePO₄ using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). It was found that the discharge capacity gradually decreased with the decrease of temperature, and the capacity at -20°C was only 33% of that at room temperature. The authors believe that with the decrease of temperature, the charge transfer impedance and Warburg impedance in the battery gradually increase, and the difference between the redox potentials in the CV curve increases. This indicates that the diffusion of lithium ions in the material slows down at low temperatures, and the kinetic rate of the Faraday reaction of the battery weakens, resulting in a significant increase in polarization (Figure 1).
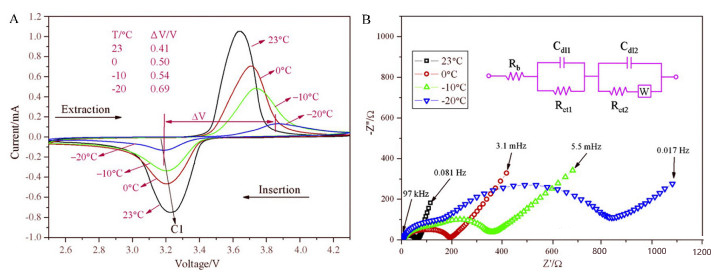
Figure 1 CV (A) and EIS (B) curves of LFP/C at different temperatures
Lv et al. designed and synthesized a composite cathode material of nickel-cobalt-manganese lithium coated with a fast ion conductor. This composite material showed excellent low-temperature performance and rate performance, maintaining a reversible capacity of 127.7 mAh·g⁻¹ at -20°C, which was much better than the 86.4 mAh·g⁻¹ of nickel-cobalt-manganese lithium material. The introduction of a fast ion conductor with excellent ionic conductivity effectively improves the Li⁺ diffusion rate, providing a new idea for improving the low-temperature performance of lithium-ion batteries.
1.2 Bulk Doping of the Material with Elements Such as Mn, Al, Cr, Mg, and F
This can increase the layer spacing of the material to improve the diffusion rate of Li⁺ in the bulk, reduce the diffusion impedance of Li⁺, and thereby enhance the low-temperature performance of the battery.
Zeng et al. prepared a carbon-coated LiFePO₄ cathode material by Mn doping. Compared with the original LiFePO₄, the polarization of the material at different temperatures was reduced to a certain extent, and the electrochemical performance of the material at low temperatures was significantly improved. Li et al. doped LiNi₀.₅Co₀.₂Mn₀.₃O₂ with Al and found that Al increased the layer spacing of the material, reduced the diffusion impedance of lithium ions in the material, and greatly improved its specific capacity at low temperatures.
The phase transition of lithium iron phosphate cathode material from lithium iron phosphate phase to iron phosphate phase during charging is slower than that from iron phosphate phase to lithium iron phosphate phase during discharging. Cr doping can promote the phase transition from iron phosphate phase to lithium iron phosphate phase during discharging, thereby improving the rate performance and low-temperature performance of LiFePO₄.
1.3 Reducing the Particle Size of the Material
This shortens the Li⁺ migration path. It should be noted that this method will increase the specific surface area of the material, thereby increasing the side reactions with the electrolyte.
Zhao et al. studied the effect of particle size on the low-temperature performance of carbon-coated LiFePO₄ materials. It was found that the discharge capacity of the material at -20°C increased with the decrease of particle size. This is because the diffusion distance of lithium ions is shortened, making the process of lithium deintercalation easier. Sun et al.’s research showed that the discharge performance of LiFePO₄ decreased significantly with the decrease of temperature, and materials with smaller particle sizes had higher capacity and discharge platforms.
2. Electrolyte
As an important part of lithium-ion batteries, the electrolyte not only determines the migration rate of Li⁺ in the liquid phase but also participates in the formation of the SEI film and plays a key role in the performance of the SEI film. At low temperatures, the viscosity of the electrolyte increases, the conductivity decreases, the impedance of the SEI film increases, and the compatibility with the positive and negative electrode materials deteriorates, which greatly impairs the energy density, cycle performance, and other properties of the battery.
At present, there are two ways to improve low-temperature performance through electrolytes:
1.Improving the low-temperature conductivity of the electrolyte by optimizing the solvent composition and using new electrolyte salts;
2.Using new additives to improve the properties of the SEI film, making it conducive to the conduction of Li⁺ at low temperatures.
2.1 Optimization of Solvent Composition
The low-temperature performance of the electrolyte is mainly determined by its low-temperature eutectic point. If the melting point is too high, the electrolyte is prone to crystallization and precipitation at low temperatures, which seriously affects the conductivity of the electrolyte. Ethylene carbonate (EC) is a main solvent component of the electrolyte, but its melting point is 36°C. At low temperatures, its solubility in the electrolyte decreases or even precipitates, which has a great impact on the low-temperature performance of the battery. By adding components with low melting points and low viscosities and reducing the content of solvent EC, the viscosity and eutectic point of the electrolyte at low temperatures can be effectively reduced, and the conductivity of the electrolyte can be improved.
Kasprzyk et al. obtained an amorphous electrolyte by mixing two solvents, EC and poly(ethylene glycol) dimethyl ether. A glass transition temperature point only appeared around -90°C. This amorphous electrolyte greatly improved the performance of the electrolyte at low temperatures; at -60°C, its conductivity could still reach 0.014 mS·cm⁻¹, providing a good solution for the use of lithium-ion batteries at extremely low temperatures.
Chain carboxylate solvents have low melting points and viscosities, and their dielectric constants are moderate, which have a good impact on the low-temperature performance of the electrolyte. Dong et al. used ethyl acetate (EA) as a co-solvent and lithium bis(trifluoromethanesulfonyl)imide as the electrolyte salt. The theoretical melting point of this electrolyte reached -91°C, and the boiling point reached 81°C. The results showed that even at an extreme low temperature of -70°C, the ionic conductivity of the electrolyte still reached 0.2 mS·cm⁻¹. Combined with an organic electrode as the positive electrode and polyimide derived from 1,4,5,8-naphthalene tetracarboxylic anhydride as the negative electrode, the battery still had 70% of the room-temperature capacity at -70°C.
Smart et al. conducted extensive research on the use of chain carboxylates as electrolyte co-solvents to improve the low-temperature performance of batteries. The research showed that using ethyl acetate, ethyl propionate, methyl acetate, and methyl butyrate as electrolyte co-solvents is beneficial to improve the low-temperature conductivity of the electrolyte and greatly improve the low-temperature performance of the battery.
2.2 New Electrolyte Salts
Electrolyte salts are an important part of the electrolyte and a key factor for obtaining excellent low-temperature performance. At present, the commercial electrolyte salt is lithium hexafluorophosphate (LiPF₆), and the SEI film formed by it has high impedance, resulting in poor low-temperature performance. The development of new lithium salts is urgent. Lithium tetrafluoroborate (LiBF₄) has a small anion radius and is easy to associate, so its conductivity is lower than that of LiPF₆. However, it has small charge transfer impedance at low temperatures and good low-temperature performance as an electrolyte salt.
Zhang et al. used LiNiO₂/graphite as electrode materials and found that the conductivity of LiBF₄ at low temperatures is lower than that of LiPF₆, but its capacity at -30°C is 86% of the room-temperature capacity, while the LiPF₆-based electrolyte is only 72% of the room-temperature capacity. This is because the LiBF₄-based electrolyte has small charge transfer impedance and small polarization at low temperatures, so the battery has good low-temperature performance. However, the LiBF₄-based electrolyte cannot form a stable SEI film at the electrode interface, resulting in severe capacity fading.
The electrolyte with lithium difluorooxalate borate (LiODFB) as the lithium salt has high conductivity under both high and low-temperature conditions, enabling lithium-ion batteries to exhibit excellent electrochemical performance over a wide temperature range. Li et al. found that the LiODFB/LiBF₄-EC/DMS/EMC electrolyte has good low-temperature performance. Tests showed that the capacity retention rate of the graphite/Li button battery after 20 cycles at 0.5 C and -20°C was: LiODFB/LiBF₄-EC/DMS/EMC (53.88%) > LiPF₆-EC/DEC/DMC/EMC (25.72%). The former has a much higher capacity retention rate than the latter, indicating that this electrolyte has a good application prospect in low-temperature environments.
As a new type of lithium salt, lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) has high thermal stability, low degree of association between cations and anions, and high solubility and dissociation degree in carbonate systems. At low temperatures, the electrolyte of the LiFSI system has high conductivity and low charge transfer impedance, ensuring its low-temperature performance. Mandal et al. used LiTFSI as the lithium salt and EC/DMC/EMC/PC (mass ratio 15:37:38:10) as the base solvent. The resulting electrolyte still had a high conductivity of 2 mS·cm⁻¹ at -40°C.
2.3 Additives
The SEI film has an important impact on the low-temperature performance of the battery. It is an ionic conductor and an electronic insulator, and is the channel for Li⁺ to reach the electrode surface from the liquid phase. At low temperatures, the impedance of the SEI film increases, and the diffusion rate of Li⁺ in the SEI film decreases sharply, which deepens the charge accumulation on the electrode surface, leads to a decrease in the lithium intercalation capacity of graphite, and enhances polarization. Improving the ionic conductivity of the SEI film at low temperatures by optimizing the composition and film-forming conditions of the SEI film is beneficial to the improvement of the low-temperature performance of the battery. Therefore, the development of film-forming additives with excellent low-temperature performance is a current research focus.
Liu et al. studied the effect of using fluoroethylene carbonate (FEC) as an electrolyte additive on the low-temperature performance of the battery. The results showed that for the graphite/Li half-cell at -20°C, the initial discharge capacity of the electrolyte with 2% FEC added was 50% higher than that of the base electrolyte at -20°C, and the charging platform was reduced by about 0.2 V. X-ray photoelectron spectroscopy (XPS) tests showed that the content of LiF in the SEI film formed by the electrolyte with FEC added was higher than that in the SEI film formed by the electrolyte without FEC added, which was beneficial to reduce the impedance of the SEI film at low temperatures, thereby improving the low-temperature performance of the battery.
Yang et al. found that the addition of LiPO₂F₂ can significantly improve the low-temperature performance of LiNi₀.₅Co₀.₂Mn₀.₃O₂/graphite pouch batteries. The capacity retention rates of the batteries with LiPO₂F₂ electrolyte after 100 cycles at 0°C and -20°C were 96.7% and 91%, respectively, while the capacity retention rates of the base electrolyte after 100 cycles were only 20.1% and 16.0%. EIS tests were performed on LiNi₀.₅Co₀.₂Mn₀.₃O₂/Li, full cells, and graphite/Li half-cells. The results showed that the addition of LiPO₂F₂ can significantly reduce the SEI film impedance and charge transfer impedance of the graphite negative electrode, and reduce polarization at low temperatures.
Liao et al.’s research showed that the addition of butyl sultone (BS) to the electrolyte is beneficial to improve the discharge capacity and rate performance of the battery at low temperatures. They used EIS, XPS, and other methods to conduct in-depth research on the mechanism of action of BS. At -20°C, after adding BS, the impedances RSEI and Rct decreased from 4094 Ω and 8553 Ω to 3631 Ω and 3301 Ω, respectively. This indicates that the addition of BS increases the charge transfer rate of lithium ions and greatly reduces polarization at low temperatures. XPS tests showed that BS is beneficial to the formation of the SEI film, which can form sulfur-containing compounds with low impedance, and at the same time reduce the content of Li₂CO₃ in the SEI film, reduce the impedance of the SEI film, and improve the stability of the SEI film.
To sum up, the conductivity of the electrolyte and the film-forming impedance have an important impact on the low-temperature performance of lithium-ion batteries. For low-temperature electrolytes, the electrolyte solvent system, lithium salt, and additives should be comprehensively optimized from three aspects. For the electrolyte solvent, a solvent system with low melting point, low viscosity, and high dielectric constant should be selected. Linear carboxylate solvents have excellent low-temperature performance, but they have a great impact on cycle performance and need to be mixed with cyclic carbonates with high dielectric constants such as EC and PC; for lithium salts and additives, the main consideration is to reduce the film-forming impedance and improve the migration rate of lithium ions. In addition, appropriately increasing the lithium salt concentration at low temperatures can improve the conductivity of the electrolyte and enhance the low-temperature performance.
3. Anode Materials
The deterioration of the diffusion kinetics of lithium ions in carbon anode materials is the primary factor limiting the low-temperature performance of lithium-ion batteries. Consequently, during the charging process, the electrochemical polarization of the anode intensifies significantly, which can easily lead to the precipitation of metallic lithium on the anode surface.
Studies by Luders et al. have shown that at -20°C, when the charging rate exceeds C/2, the amount of precipitated metallic lithium increases remarkably. At a charging rate of C/2, the amount of lithium precipitated on the anode surface accounts for approximately 5.5% of the total charging capacity; however, this proportion rises to 9% at a rate of 1C. The precipitated metallic lithium may further develop and eventually form lithium dendrites. Therefore, when batteries must be charged at low temperatures, it is necessary to use the smallest possible current for charging. Additionally, after charging, the lithium-ion batteries should be allowed to stand sufficiently. This ensures that the metallic lithium precipitated on the anode can react with graphite and be re-intercalated into the interior of the graphite anode.
Zinth et al. employed techniques such as neutron diffraction to conduct a detailed study on the lithium plating behavior of NMC111/graphite 18650-type lithium-ion batteries at a low temperature of -20°C. The batteries were charged and discharged following the process illustrated in Figure 2. Figure 3 presents a comparison of the phase changes in the graphite anode when charged at rates of C/30 and C/5, respectively.
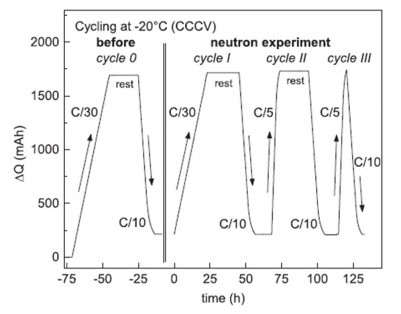
Figure 2 Relationship between ΔQ and time during the charge-discharge process at a low temperature of -20°C in the neutron diffraction experiment
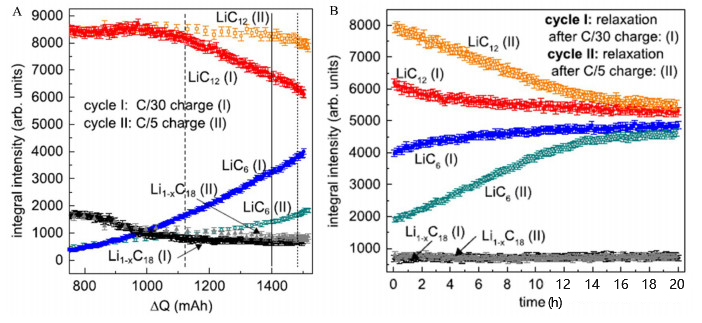
Figure 3 Comparison of phase changes in the anode after charging at different rates (A) and after standing for 20 hours (B)
As shown in the figure, for the two different charging rates, the lithium-deficient phase Li₁₋ₓC₁₈ is very similar, with the main differences observed in the two phases LiC₁₂ and LiC₆. In the early stage of charging, the phase change trends in the anode are relatively similar under the two charging rates. For the LiC₁₂ phase, when the charging capacity reaches 95 mAh, the change trends begin to diverge; when the capacity reaches 1100 mAh, a significant difference in the LiC₁₂ phase emerges between the two rates. At the low charging rate of C/30, the LiC₁₂ phase decreases very rapidly, while at the C/5 rate, the decrease rate of the LiC₁₂ phase is much slower. This indicates that due to the deteriorated lithium intercalation kinetics of the anode at low temperatures, the rate of further lithium intercalation into LiC₁₂ to form the LiC₆ phase is reduced. Correspondingly, the LiC₆ phase increases very quickly at the low C/30 rate but much more slowly at the C/5 rate. This suggests that at the C/5 rate, less Li is intercalated into the crystal structure of graphite. However, the charging capacity of the battery at the C/5 rate is slightly higher than that at the C/30 rate. The excess Li that is not intercalated into the graphite anode is most likely precipitated on the graphite surface in the form of metallic lithium, which is indirectly supported by the standing process after the end of charging.
Zhang et al. used the EIS method to measure the variation trends of the impedance parameters (Re, Rf, and Rct) of graphite/Li half-cells with temperature. They found that all three parameters increase as the temperature decreases, where the increase rates of Re and Rf are roughly the same, while the increase rate of Rct is much faster. When the temperature drops to -20°C, Rct has become the main component of the total battery impedance. This indicates that the deterioration of electrochemical reaction kinetics is the primary factor causing the decline in low-temperature performance.
Selecting appropriate anode materials is a key factor in improving the low-temperature performance of batteries. Currently, the optimization of low-temperature performance is mainly achieved through approaches such as anode surface treatment, surface coating, doping to increase interlayer spacing, and controlling particle size.
(1) Surface Treatment
Surface treatment includes surface oxidation and fluorination. It can reduce the active sites on the graphite surface, lowering irreversible capacity loss. Meanwhile, it can create more micro-nano structural pores, which are beneficial for Li⁺ transport and impedance reduction.
Zhang Lijin et al. treated graphite with oxidative micro-expansion. After the treatment, the average grain size of graphite decreased, the amount of Li⁺ intercalated on the surface and edges of carbon layers increased, and the nano-scale pore structure introduced on the graphite surface further expanded the Li⁺ storage space. Wu et al. used 5 at% fluorine gas to fluorinate natural graphite at 550°C; the electrochemical performance and cycle performance of the treated material were significantly improved.
(2)Surface Coating
Surface coatings (e.g., carbon coating, metal coating) can not only avoid direct contact between the anode and the electrolyte, improving the compatibility between the electrolyte and the anode, but also enhance the electrical conductivity of graphite and provide more lithium intercalation sites, thereby reducing irreversible capacity. In addition, the interlayer spacing of soft carbon or hard carbon materials is larger than that of graphite. Coating a layer of soft carbon or hard carbon on the anode is conducive to Li⁺ diffusion and reduces SEI film impedance, thus improving the low-temperature performance of the battery. Surface coating with a small amount of Ag improved the conductivity of the anode material, endowing it with excellent electrochemical performance at low temperatures.
Li et al. developed an Fe/Fe₃C-CNF composite material with good low-temperature performance, which retained a capacity of 250 mAh·g⁻¹ after 55 cycles at -5°C. Ohta et al. studied the effect of different anode materials on the performance of lithium-ion batteries. They found that both carbon-coated artificial graphite and carbon-coated natural graphite had significantly lower irreversible capacity compared to uncoated graphite. At the same time, the carbon-coated graphite anode could effectively improve the low-temperature performance of the battery: the graphite with a 5% coating amount maintained 90% of its room-temperature discharge capacity at -5°C. Nobili et al. used tin-coated graphite as the anode material; at -20°C, the SEI film impedance and charge transfer impedance of the coated material were reduced by 3 times and 10 times, respectively, compared to the uncoated material. This indicates that tin coating can reduce the polarization of the battery at low temperatures, thereby improving its low-temperature performance.
(3) Increasing Graphite Interlayer Spacing
The interlayer spacing of graphite anodes is small. At low temperatures, the diffusion rate of Li⁺ between graphite layers decreases, leading to increased polarization. During graphite preparation, introducing elements such as B, N, S, and K can modify the graphite structure, increase its interlayer spacing, and improve its lithium deintercalation/intercalation capability. The atomic radius of P (0.106 pm) is larger than that of C (0.077 pm); doping P can increase the interlayer spacing of graphite, enhance Li⁺ diffusion capacity, and may also increase the content of graphite microcrystals in carbon materials. When K is introduced into carbon materials, the intercalation compound KC₈ is formed; after K is extracted, the interlayer spacing of the carbon material increases, which is conducive to the rapid intercalation of Li and thus improves the low-temperature performance of the battery.
(4) Controlling Anode Particle Size
Huang et al. studied the effect of anode particle size on low-temperature performance. They found that coke anodes with average particle sizes of 6 μm and 25 μm exhibited the same reversible charge-discharge capacity at room temperature. However, at -30°C, the coke electrode with a particle size of 25 μm could only deliver 10% of its room-temperature capacity, while the coke electrode with a particle size of 6 μm could deliver 61% of its room-temperature capacity.
From this experimental result, it can be concluded that the larger the anode particle size, the longer the Li⁺ diffusion path and the greater the diffusion impedance, leading to increased concentration polarization and deteriorated low-temperature performance. Therefore, appropriately reducing the particle size of the anode material can effectively shorten the migration distance of Li⁺ between graphite layers, reduce diffusion impedance, and increase the electrolyte wettability area, thereby improving the low-temperature performance of the battery. In addition, graphite anodes prepared by granulating single small-sized particles have high isotropy, which can provide more lithium intercalation sites, reduce polarization, and also significantly improve the low-temperature performance of the battery.
4. Conclusions
In summary, the low-temperature performance of lithium-ion batteries is a key factor restricting the application of lithium batteries, and how to improve the low-temperature performance of lithium batteries remains a current research focus and challenge.
The reaction process of the battery system mainly includes four steps: Li⁺ transport in the electrolyte, Li⁺ migration across the electrolyte/electrode interface film, charge transfer, and Li⁺ diffusion in the bulk of the active material. At low temperatures, the rate of each step decreases, which increases the impedance of each step, intensifies electrode polarization, and causes problems such as reduced low-temperature discharge capacity and lithium plating on the anode.
Improving the low-temperature performance of lithium batteries should comprehensively consider the effects of various factors in the battery, including the cathode, anode, and electrolyte. Specifically, the conductivity of the electrolyte should be improved by optimizing the composition of the electrolyte solvent, additives, and lithium salts, while reducing the film-forming impedance; the cathode and anode materials should be modified through doping, coating, and particle size reduction to optimize the material structure and reduce the interface impedance and the diffusion impedance of Li⁺ in the bulk of the active material. Through the overall optimization of the battery system, the polarization of lithium batteries at low temperatures is reduced, and the low-temperature performance of the batteries is further improved.